The World of Zinc Primers Just Changed
Originally Published: BIC, June/July 2018
How can something that has been the same for over 50 years now be greatly improved? New technology developed now allows zinc primers to be applied at thicker films, which provides more protection. The world of zinc primers just changed for the better.
There is nothing new about the advantages of using zinc-rich primers to protect carbon steel from corrosion. History reveals that the first zinc-rich primers with zinc particles held together with a polymer were utilized to protect steel back in the 1930s, more than 80 years ago. Perhaps the last major breakthrough in zinc-rich primer technology was nearly 56 years ago when Carboline patented Carbozinc 11. This revolutionized the industry by significantly speeding up the coating process while providing legendary corrosion protection for the steel. In fact, many popular steel structures are still being protected today with a single coat of inorganic zinc primer. It is believed that zinc-rich primer will protect the steel for the life of the structure. We, as an industry, have continued to develop zinc primer technology to expand its use. We have made improvements in curing properties to expand the use of zinc primers into more varied climates and applications. Lowering volatile organic compound (VOC) and hazardous air pollutant (HAP) levels has helped improve environmental effects during application. Improvements in weldability have allowed structural steel to be protected during the construction phase while not hindering the welding process. The resin systems used have also been expanded beyond inorganic silicates to include epoxy, urethane, and others, all of which offer similar benefits and can have similar issues.
You may ask, “What is the big deal with zinc-rich primers? Why are zinc-rich coatings one of the best ways to protect steel?” First, we must understand that metal corrosion is defined as the deterioration by chemical or electrochemical reaction resulting from exposure to weathering, moisture, chemicals or other agents in the environment in which it is placed. This reaction causes refined metals such as steel to return to their original, natural form, which is iron oxide or rust. Zinc-rich coatings work by providing galvanic corrosion protection the same way galvanizing protects a metal fence or zinc anodes protect a ship hull. To summarize, zinc is less noble or more reactive than steel. When zinc and steel are in contact together within an electrolyte (water), the zinc will sacrifice itself and corrode to protect the steel. This is called cathodic protection. In coatings, this is accomplished by placing metallic zinc dust in a polymer that is in direct contact with the steel. The less noble zinc dust serves as an anode, sacrificing itself through the flow of electrons to protect the more noble steel. Therefore, the steel does not corrode.
Zinc-rich coatings also provide an additional benefit in that they are somewhat “self-healing.” If there is a small break in the coating, zinc oxides, zinc hydroxide, zinc carbonate or zinc salts form a protective layer that helps to prevent moisture (electrolyte) from reaching the steel and causing corrosion.
You may ask, “If zinc is so good, then why shouldn’t we put on more?” If there is one main disadvantage to these traditional zinc-rich coatings, it is that they must be applied at relatively low dry film thickness (DFT), typically less than 5 mils. This is approximately the thickness of one sheet of 24-pound copy paper. If these zinc-rich primers are applied at higher thicknesses, the result is often cracks in the coating and/or solvent entrapment, both of which may lead to premature coating failure and ultimately, the formation of corrosion. The low DFT of zinc primers can make them more susceptible to mechanical damage that may result from impact and abrasion during typical shipping and handling, erecting structures, etc. Zinc-rich coatings that have been applied to structures, vehicles, workboats, barges, ships, etc. are also often damaged by accidental contact with other objects while in service. If the mechanical damage is severe enough, the coating may not be able to “self-heal,” and the result is often corrosion. The corrosion protection available is due to the level of zinc present, which is a function of zinc primer thickness. None of the existing zinc primers in use today allow high film thickness application.
Extensive research on resin systems, pigments, and additives has led to the development of a unique zinc primer that can be applied at 5-10 mils DFT, more than twice the thickness of traditional zinc primers, without cracking or solvent entrapment. In the high-performance coatings industry, more primer equals better protection.
This revolutionary coating design uses a phenalkamine epoxy resin as the polymer binder, combined with a specially engineered blend of both structural and functional pigments. The pigment package is designed to provide strength to the coating film while allowing the zinc to perform its protective function. Various pigment types, forms, and sizes were selected, and their use optimized in the formulation to yield the best performance possible. The phenalkamine epoxy binder provides many advantages, including excellent adhesion and durability, fast-cure to handle and topcoat, low-temperature cure, and tolerance to moisture during application and cure, to name a few. This unique combination provides a thick 5-10 mil DFT reinforced film that provides outstanding galvanic corrosion protection, combined with excellent impact and abrasion resistance to help resist mechanical damage.
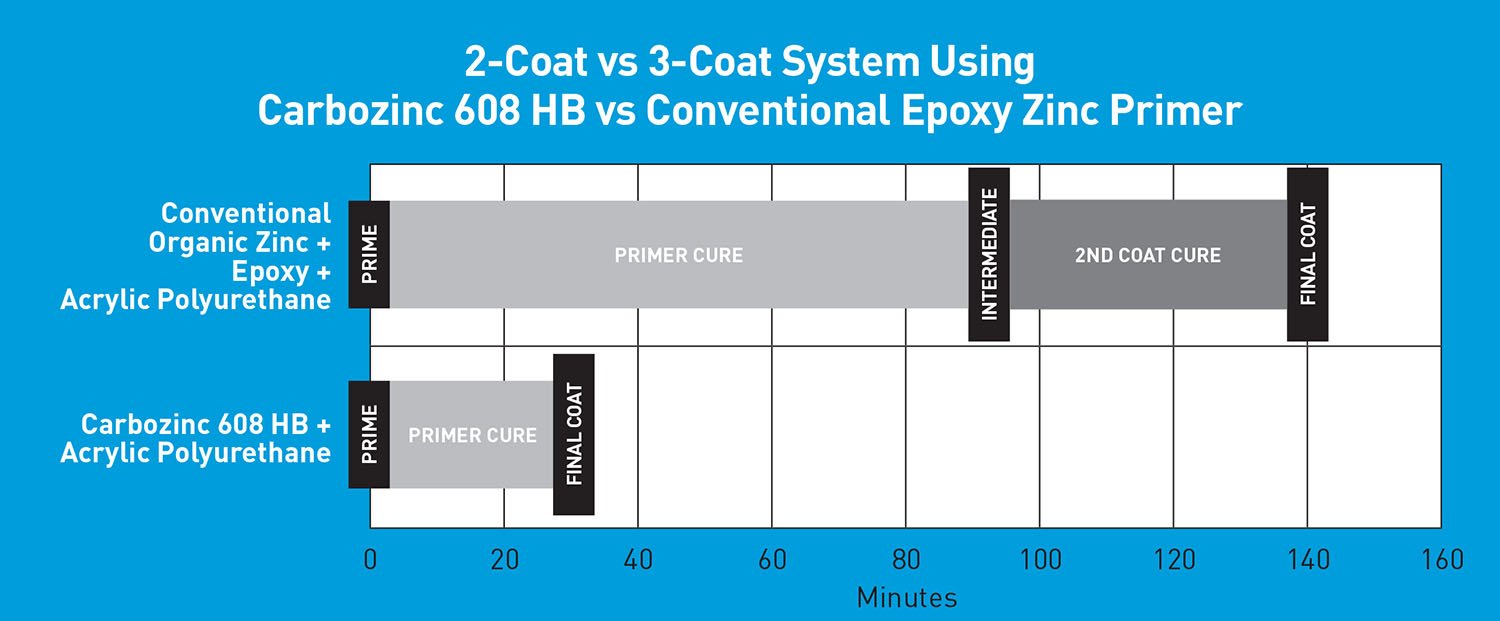
Why does more primer equal better corrosion protection? Typically, the primer provides the majority of the corrosion protection within a coating system. In the past, the best combination of corrosion protection and aesthetics (good long-term color and gloss retention in UV exposure) has been achieved by specifying one coat of zinc-rich primer at 3-5 mils DFT, one intermediate coat of epoxy at 4-6 mils DFT, and one finish coat of aliphatic polyurethane at 2-3 mils DFT. The overall thickness of this typical three-coat system is approximately 9-14 mils DFT. In an effort to save time and money, many steel projects have been coated with only two coats instead of the traditional three coats. This concept of eliminating the epoxy intermediate coat is not new. It typically involves using a zinc-rich epoxy coating applied at 3-5 mils DFT, with a single thicker coat of high-build aliphatic polyurethane or polysiloxane topcoat applied at 3-7 mils DFT. The total thickness of these traditional two-coat systems ranges from 10-13 mils DFT, which is similar to the total thickness of traditional three-coat systems. However, all of these traditional systems still utilize only 3-5 mils DFT of zinc-rich primer, which must provide the majority of the corrosion protection.
A zinc primer of 5-10 mils DFT followed by an esthetic topcoat of 3-7 mils DFT can be a much better option. This system can provide comparable or greater overall thickness with up to twice as much zinc primer, which provides the majority of the corrosion protection. More zinc primer was not an option before, but it is now as a result of persistent research efforts.
In the fast-paced world that we live in today, the old adage, “Time is money,” is probably more important now than ever before. It is also true that the cost of building new steel assets is higher now than ever before. Therefore, we need to preserve our assets with the best methods available to provide the longest service life possible. Using a two-coat system in lieu of three coats offers significant savings in both time and money. A new primer technology is now available that allows the use of a two-coat system while providing up to 10 mils DFT of a durable zinc-rich primer. Using this primer in a two-coat system provides twice the thickness of traditional zinc primer to help protect your assets longer. More zinc primer will provide more protection.
By: Gus Badalamenti, Carboline Company

